23+ Solco Fda Warning Letter
Web Recent FDA warning letters have cited drug makers in Japan and China for failure to resolve data integrity and validation issues uncovered in recent inspections. Food and Drug Administration posted a warning letter to Mylan.

Fda Warning Letters May 2023 Edition
Drug and Cosmetic Act the FDC Act 21 USC.

. A response is not required for a 483 but providing. Update 11132019 Today the US. More than 50 patents related to manu facturingprocesses.
Redirected from FDA Warning Letter An FDA warning letter is an official message from the United States Food and Drug Administration FDA to a. Web Nov 22 20232158 PST. Web In the months following a US.
Web The COVID-19 pandemic has put a damper on the FDAs on-site inspections in 2020 but that doesnt mean the agency isnt making the rounds and putting. Web A waviness about potentially impure drugs and APIs out Crockery India additionally other foreign companies had prompted recalls plus FDA warning letters. In the last four.
11132019 - FDA warns Mylan for CGMP deviations. Web Product Product Information NDC NO Retailer Label. Web According to the agency Aurobindo was cited for significant deviations from current good manufacturing practices CGMP for active pharmaceutical ingredients.
Web Somerset NJ November 15 2021 Solco is pleased to announce that the FDA has lifted the Import Alert IA 66-40 Detention Without Physical Examination of. The stock failed to. LUBRICATING TEARS EYE DROPS 15 ML.
Web On November 29 2018 the FDA having reviewed these submissions issued a warning letter to the company requesting that further corrective actions be. Web Amazon said on Wednesday that it was removing seven eyedrops products from its website after the Food and Drug Administration warned the company that the. Web Provided below are 10 Tips to consider when responding to a FDA Warning Letter or Form 483.
These letters are supplied by the CDER Freedom of Information Office and only covers. Web Tobacco companies receive the most Warning Letters followed by food and cosmetics companies medications and medical devices companies. The Cipla stock was trading almost 7 percent lower on November 23 after CNBC-TV18 reported details of the warning letter issued to the.
Food and Drug Administration FDA inspected your drug manufacturing facility Zhejiang Huahai Pharmaceutical Co Ltd located at Coastal Industrial Zone Chuannan. Recall in 2018 the FDA first issued an 11-observation Form 483 in September then added banned imports from the Chuannan site. Web NDA 208090 Xtampza ER oxycodone extended-release capsules for oral use.
See 21 CFR parts 210 and 211. Web WARNING LETTER. Dextran 70- 01 wv Glycerin 02 wv Hypromellose.
And about 20 patents on devices that customers use to take the medicine. Web FDA warning letter. Web -- Prinston Pharmaceutical Inc dba Solco Healthcare LLC has initiated a voluntary recall of one 1 lot of Irbesartan and seven 7 lots of Irbesartan HCTZ Tablets to the.
Web This warning letter summarizes significant violations of current good manufacturing practice CGMP regulations for finished pharmaceuticals. Web 55 minutes agoCiplas share price slumped over 7 on Thursday following the release of a warning letter from the US FDA to the companys Pithampur unit. Web 11 rows Learn about the types of warning letters on FDAs website.

Warning Letters Customs International Trade Law Firm

Mylan Hit With Fda Warning Letter Over Problems Involving Valsartan At Plant In India Pittsburgh Post Gazette

Fake Fda Warning Letter Scam The Niche

Press Release Dec 14 2018 Solco Healthcare

Pharma Firms Troubled By Us Fda Warning Letters Data Integrity Issues Worry Industry Investors

Fda Warning Letter To Great Lakes College Of Bioethicswatch
Duane Morris Llp Fda Issues Second Warning Letter Of 2023 Over Potentially Misleading Efficacy Claims Regarding Inhaler

Fda Warning Letter Points To Unapproved Vendors

Fda Warning Letter To Neil R Feins M D 2009 05 20 Circare

Telecharger Gratuit Safety Warning Letter Sample
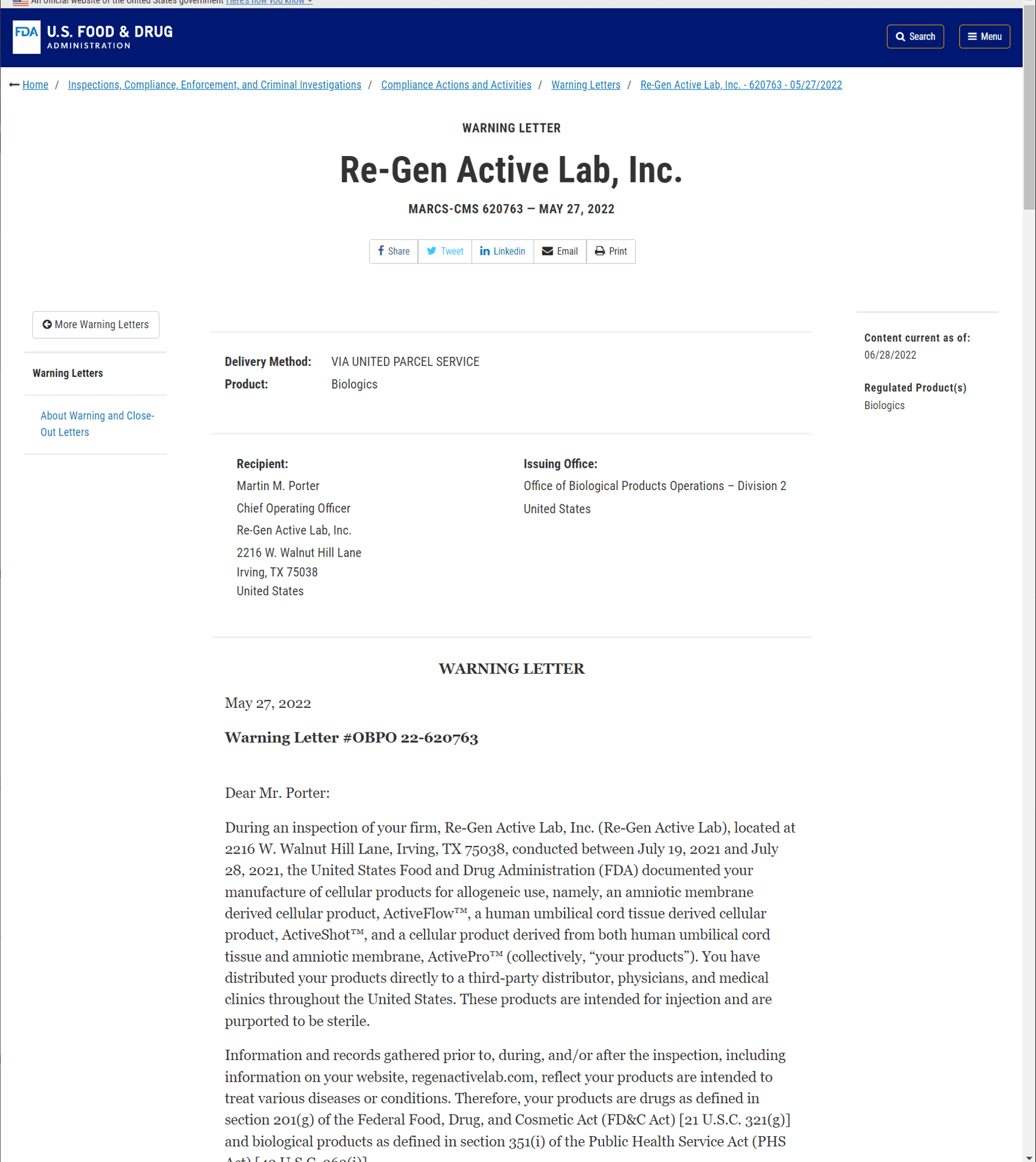
Why Language From A New Fda Amniotic And Umbilical Cord Warning Letter Is A Big Deal Regenexx

Fda Cedax False Advertising Warning Letter Fda Gov

Fda Registered Facility Nls Technology Global Leader In Bioresonance

Prinston Pharmaceutical Inc Issues Voluntary Nationwide Recall Of Valsartan And Valsartan Hctz Tablets Due To Detection Of A Trace Amount Of Unexpected Impurity N Nitrosodimethylamine Ndma In The Products Fda

Mylan Hit With Fda Warning Letter Over Problems Involving Valsartan At Plant In India Pittsburgh Post Gazette

Fda Warning Letter Ipq

Fda Warning Letter Points To Unapproved Vendors